|
|
Name: |
Yue Jianmin
|
Education: |
Ph.D
|

|
Positions: |
Director of Department of Natural Products Chemistry,PI,Director of Department of Natural Medicinal Chemistry,Deputy Dirctor of Chinese National Compound Library
|
Academic title: |
Professor;Academician of CAS
|
Phone: |
021-50806718
|
Fax: |
021-50806718
|
E-mail: |
jmyue@simm.ac.cn
|
Personal Website: |
|
Postal Code: |
201203
|
Mailing Address: |
555 Zu Chong Zhi Road , Zhang Jiang Hi-Tech Park, Pudong, Shanghai
|
|
| Resume: |
|
EDUCATION
1980.09 – 1984.07 Department of Chemistry, Lanzhou University, BSc.
1984.09 – 1987.07 Department of Chemistry, Lanzhou University, MSc.
1987.09 – 1990.07 Department of Chemistry, Lanzhou University, PhD.
WORK EXPERIENCE
1990.07 – 1993.09 Postdoctoral Fellow, Department of Phytochemistry, Kunming Institute of Botany, Chinese Academy of Sciences; Supervisor: Prof. Handong Sun.
1993.09 – 1994.09 Postdoctoral Fellow, School of Chemistry, University of Bristol, UK; Supervisor: Prof. Geoffrey Eglinton.
1994.09 – 1996.04 Associate Professor, Department of Phytochemistry, Kunming Institute of Botany, Chinese Academy of Sciences.
1996.05 – 1999.04 Senior Research Scientist and project leader, Joint Lab of Unilever Research and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.
1999.05 – Present Professor, Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
2011.09 – 2011.11 Visiting Professor, Department of Chemistry, The University of Queensland, Australia.
2002.01 – 2002.02 Visiting Professor, Novartis Pharma AG, Switzerland.
|
| Research Directions |
|
1. Extraction, isolation, and structural characterization of novel bioactive constituents from natural sources especially traditional Chinese medicine and folk medicine.
2. Discovery of drug leads and candidates from natural products.
3. Structural modification and total synthesis of natural compounds with significant bioactivities.
|
| Social Titles |
|
Prof. Yue is the associate editor or editorial board member of four professional journals, Natural Products and Bioprospecting, Journal of Chinese Pharmaceutical Sciences, Journal of Asian Natural Products Research, and Chinese Journal of Natural Medicines.
|
| Awards & Honors |
|
2010 The first prize of Shanghai Municipal Award in natural science
2013 The second prize of State Natural Science Award
|
| Achievements |
|
1. Recent work in the research of Daphniphyllum alkaloids with diverse complex structures and unique biosynthetic pathways continue to attract attention from many researchers in the last five year. There have been continuously new advancements and reports from areas of both natural products and organic synthesis. We have intensively studied the alkaloidal constituents of five Daphniphyllum plants and obtained over 40 Daphniphyllum alkaloids including 12 new ones. Of the new alkaloids, calycinumine A (1), angustimine (2) and logeracemin A (3) are with new carbon frameworks. With a unique spiro ring system, logeracemin A (3) represents the first dimeric alkaloid of this structural family and its absolute structure was determined via spectroscopic data, X-ray diffraction analysis and computational ECD calculation. Most excitingly, 3 exhibited significant anti-HIV activity with an IC50 of 4.5 ± 0.1 mM (SI = 6.2). This discovery was published in the top chemical journal J. Am. Chem. Soc., and represents a milestone work in the studies of Daphniphyllum alkaloids.
2. Discovery of novel macrolides as new type of immunosuppressants Khaya ivorensis is mainly distributed in the tropical countries of Africa, such as Cameroon, Angola, and Nigeria, and is also cultivated in the Southern provinces of China. Our current investigation of the non-polar fraction of the ethanolic extract of its stem bark has led to the isolation and identification of two novel macrolides, ivorenolides A (4) and B (5) with 18- and 17-memebered heterocycles, respectively. Compounds 4 and 5 both incorporate oxane and conjugated acetylene functionalities (rare in plant metaboites). The absolute configuration of 4 was established via spectroscopic methods, X-ray crystallography and total synthesis of its enantiomer, while that of 5 was elucidated through spectroscopic data and total synthesis of its four most likely stereoisomers. Our biological tests demonstrated that 4 and its enantiomer displayed decent inhibition against ConA induced murine T-cell and LPS induced murine B-cell proliferation. In addition, the selective indexes of 4 and its enantiomer are equivalent to or even better than those of the positive controls (CsA & PSA). These new outcomes were published in J. Am. Chem. Soc. and Org. Lett., respectively.

Figure 1. Representative Daphniphyllum alkaloids
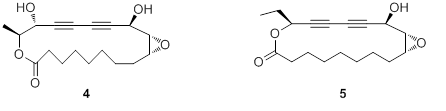
Figure 2. Structures of macrolides from Khaya ivorensis
3. New advances in the study of Meliacious limonoids
The Meliacious plants are well-known for their rich limonoid constituents with diverse and complex structures, and some limonoids have been reported to possess antifeeding, antimalarial and neuroprotective activities. The limonoid structure family has always been a hot research topic in natural products field because of their abundant complex carbon frameworks and biological properties. There are 62 species and 12 varieties belonging to 15 genera distributed in the tropical and subtropical areas of China. On the basis of our previous brilliant research work of the Meliacious limonoids, we have devoted lots of efforts to the investigations on 20+ Meliacious plants in the last five years. This five-year program has returned nearly 400 compounds comprising >150 new ones and more than half of these isolates are limonoids. Different types of limonoids showed antimicrobial, potassium channel inhibitory and 11?-HSD1 inhibitory activities. The new representative structures with new architectures are shown in Figure 3, such as chukrasone B (7) with an unprecedented 16,19-dinorlimonoid skeleton and walsucochinoid A (9) with a novel highly rearranged and aromatized ring-D motif.
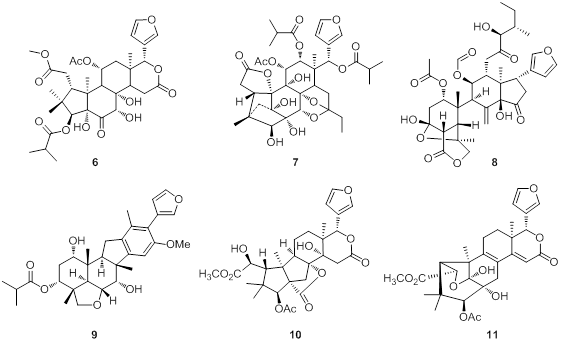
Figure 3. Representative Meliacious limonoids
4. New advances in the study of terpenoid compounds
In addition to limonoids, the study of other terpenoid constituents from TCM is also our main research content. We have investigated the terpenoid compounds from a number of species of the Meliaceae, Myrtaceae, Euphorbiaceae and Chloranthaceae families, and this has proved to be a very rewarding program.
(a) Investigation of diterpenoids The chemical study of Aphanamixis grandifolia have returned four linear diterpenoids with new carbon skeleton, represented by aphadilactone C (12). The absolute structures of them were established on the basis of spectroscopic data, chemical degradation, fragment synthesis, and calculated ECD data. Our biological tests determined that compound 12 is a potent (IC50 = 0.46 ?M) and highly selective (SI >217) diacylglycerol O-acyltransferase-1 inhibitor, being the strongest natural DGAT-1 inhibitor up to date. Meanwhile, the four new diterpenoids also exhibited remarkable antimalarial activities against Plasmodium falciparum (IC50 0.12 to 1.35 ?M). Two new diterpenoids (e.g. 13) with a new rearranged ent-clerodane backbone were obtained and identified from the medicinal plant Croton laevigatus, with their absolute configurations being established via X-ray experiments. This is one of the few recent reports on diterpenoids with new frameworks. We have intensively studied the chemical constituents of four Trigonostemon species in the recent 5 years, and discovered over 40 daphnane-type diterpenoids. Some of these compounds showed decent cytotoxicities against two human tumor cell lines HL-60 and A-549. One of the representative structures is trigochilide B (14) that features a new 12-carbon fragment to form a novel macrolide functionality with three conjugated double bonds.
(b) Investigation of sesquiterpenoids Plants of the Chloranthaceae family are well-known for their medicinal values and secondary metabolites of lindenane-type sesquiterpenoids and corresponding dimers. Our current work of Chloranthus serratus, a TCM, has reported for the first time two new-skeleton sesquiterpenoid dimer (e.g. 15) derived from an elemane and a eudesmane. The absolute structures of them were characterized via spectroscopic data, X-ray diffraction analyses, and calculated ECD data. We have obtained three sesquiterpenoid derivatives with mixed biogenesis from Eucalyptus globulus of the Myrtaceae family. Eucalyptin A (16) is the representative compound with new a carbon framework. All of the three meroterpenoids showed significant inhibition against HGF/c-Met axis which is a hot cancer target of recent years, representing a new class of c-Met inhibitors. Preliminary structure-activity relationship study revealed that neither separate sesquiterpenoid nor mere simple phloroglucinol derivative showed inhibitory effect, demonstrating that the coupling of the two structural units are necessary for their activities.

Figure4. Representative terpenoid compounds
|
| Grants & Research Projects |
|
1. National Natural Science Foundation, Discovery and Study of New Bioactive Agents from Important Dai Medicine with Efficacy of Clearing Heat and Toxins, Project leader, 2014.01-2017.12
2. National Natural Science Foundation, Discovery and Functional Study of New Mexicanolide type Limonoids, Project leader, 2013.01-2016.12
3. Ministry of Science and Technology, Modification and Systematic Optimization of Metabolically Engineered Yeast, Program leader, 2012.01-2016.8
4. Ministry of Science and Technology, Construction of Goingtoscale Library of TCM Chemical Constituents, Principal Investigator, 2011.01-2013.12
5. Ministry of Science and Technology, Natural Products Platform, Principal Investigator, 2009.01-2011.12
6. National Natural Science Foundation, Natural Medicinal Chemistry, Project leader, 2012.01-2016.12
7. National Natural Science Foundation, Investigation of the Bioactive Chemicals from Three Walsura species, Project leader, 2013.01-2016.12
8. National Natural Science Foundation, Study of the Chemical Constituents and Their Bioactivities from Three Aglaia Species, Project leader,2012.01-2014.12
|
| Pubilcations |
|
(2009.1.1-)
1. Xing Yan, Yun Fan, Wei Wei, Pingping Wang, Qunfang Liu, Yongjun Wei, Lei Zhang, Guoping Zhao*, Jianmin Yue*, Zhihua Zhou*, Production of bioactive ginsenoside compound K in metabolically engineered yeast, Cell Research, 2014, 24, 770-773.
2. Jin-Biao Xu, Hua Zhang, Li-She Gan, Ying-Shan Han, Mark A Wainberg, and Jian-Min Yue*, Logeracemin A, an Anti-HIV Daphniphyllum Alkaloid Dimer with a New Carbon Skeleton from Daphniphyllum longeracemosum, Journal of the American Chemical Society, 2014, 136(21), 7631-7633.
3. Yao Wang, Quan-Fang Liu, Ji-Jun Xue, Yu Zhou, Huang-Chao Yu, Sheng-Ping Yang, Bo Zhang, Jian-Ping Zuo, Ying Li*, and Jian-Min Yue*,Ivorenolide B, an Immunosuppressive 17-Membered Macrolide from Khaya ivorensis: Structural Determination and Total Synthesis, Organic Letters, 2014, 16 (7), 2062-2065.
4. Jia Liu, Xiu-Feng He,Gai-Hong Wang, Emilio F. Merino, Sheng-Ping Yang, Rong-Xiu Zhu, Li-She Gan, Hua Zhang, Maria B. Cassera, He-Yao Wang, David G. I. Kingston, and Jian-Min Yue*, Aphadilactones A?D, Four Diterpenoid Dimers with DGAT Inhibitory and Antimalarial Activities from a Meliaceae Plant,The J. of Organic Chemistry, 2014, 79(1), 599-607.
5. Hua Zhang, Hong-Bing Liu, and Jian-Min Yue*, Organic Carbonates from Natural Sources, Chemical Reviews, 2014, 114 (1), 883-898.
6. Guo-Cai Wang, Hua Zhang, Hong-Bing Liu, Jian-Min Yue*, Laevinoids A and B: Two Diterpenoids with an Unprecedented Backbone from Croton laevigatus, Organic Letters, 2013, 15(18), 4880-4883.
7. Hua Zhang, Chuan-Rui Zhang, Kong-Kai Zhu, An-Hui Gao, Cheng Luo, Jia Li, and Jian-Min Yue*, Fluevirosines A?C: a Biogenesis Inspired Example in the Discovery of New Bioactive Scaffolds from Natural Sources, Organic Letters, 2013, 15 (1), 120-123.
8. Bo Zhang, Yao Wang, Sheng-Ping Yang, Yu Zhou, Wen-Bin Wu, Tang Wei, Jian-Ping Zuo, Ying Li* and Jian-Min Yue*, Ivorenolide A, an Unprecedented Immunosuppressive Macrolide from Khaya ivorensis: Structural Elucidation and Bioinspired Total Synthesis, Journal of the American Chemical Society 2012, 134(51), 20605-20608.
9. Sheng-Ping Yang, Xiao-Wei Zhang, Jing Ai, Jin-Biao Xu, Bo Zhang, Zu-Shang Su, Ying Wang, Lu Wang, Jian Ding, Mei-Yu Geng*, and Jian-Min Yue,* Potent HGF/c-Met Axis Inhibitors from Eucalyptus globules: the Coupling of Phloroglucinol and Sesquiterpenoid Is Essential for the Activity,Journal of Medicinal Chemistry, 2012, 55(18), 8183-8187.
10. Hong-Bing Liu, Hua Zhang, Ping Li, Zhao-Bing Gao, and Jian-Min Yue*, Chukrasones A and B: Potential Kv1.2 Potassium Channel Blockers with New Skeletons from Chukrasia tabularis, Organic Letters, 2012, 14(17), 4438-4441.
11. Tao Yuan, Rong-Xiu Zhu, Sheng-Ping Yang, Hua Zhang, Chuan-Rui Zhang, Jian-Min Yue*, Serratustones A and B Representing an Unprecedented Dimerization Pattern of Two Types of Sesquiterpenoids from Chloranthus serratus, Organic Letters, 2012, 14(12), 3198-3201.
12. Mei-Ling Han, Hua Zhang, Sheng-Ping Yang and Jian-Min Yue*, Absolute Structures of Walsucochinoids A and B: Novel Rearranged Limonoids from Walsura cochinchinensis, Organic Letters, 2012, 14(2), 486-489.
13. Chuan-Rui Zhang, Cheng-Qi Fan, Shi-Hui Dong, Hong-Bing Liu, Wen-Bin Zhou, Yan Wu, and Jian-Min Yue*, Angustimine and Angustifolimine: Two New Alkaloids from Daphniphyllum angustifolium, Organic Letters, 2011, 13(9), 2440-2443.
14. Sheng-Ping Yang, Hua-Dong Chen, Shang-Gao Liao, Bo-Jun Xie, Ze-Hong Miao, and Jian-Min Yue*, Aphanamolide A, a New Limonoid from Aphanamixis polystachya, Organic Letters, 2011, 13(1), 150-153.
15. Hua-Dong Chen, Sheng-Ping Yang, Xiu-Feng He, Jing Ai, Zhen-Kai Liu, Hong-Bing Liu, Mei-Yu Geng, and Jian-Min Yue*, Trigochinins A?C: Three Novel Daphnane-type Diterpenes from Trigonostemon chinensis, Organic Letters 2010, 12(6), 1168-1171.
16. Tao Yuan, Rong-Xiu Zhu, Sheng-Ping Yang, Shang-Gao Liao and Jian-Min Yue?,Structure Determination of Grandifotane A from Khaya grandifoliola by NMR, X-Ray Diffraction, and ECD Calculation, Organic Letters, 2010, 12(2), 252-255.
17. Shang-Gao Liao, Hua-Dong Chen and Jian-Min Yue*,Plant Orthoesters, Chemical Reviews, 2009, 109(3), 1092-1140.
18. Chuan-Rui Zhang, Hong-Bing Liu, Shi-Hui Dong, Jian-Yong Zhu, Yan Wu, and Jian-Min Yue*, Calycinumines A and B, two novel alkaloids from Daphniphyllum calycinum, Organic Letters, 2009, 11(20), 4692-4695.
19. Hua-Dong Chen, Xiu-Feng He, Jing Ai, Mei-Yu Geng and Jian-Min Yue*, Trigochilides A and B, Two Highly Modified Daphnane-Type Diterpenoids from Trigonostemon chinensis, Organic Letters, 2009, 11(18), 4080-4083.
20. Tao Yuan, Sheng-Ping Yang, Chuan-Rui Zhang, Sheng Zhang, and Jian-Min Yue*, Two Limonoids, Khayalenoids A and B with an Unprecedented 8-Oxa-tricyclo[4.3.2.02,7] undecane Motif from Khaya senegalensis, Organic Letters, 2009, 11(3), 617-620.
|
|
|
|
|
|
|
|